Assigning Oxidation States in Challenging Transition Metal Complexes
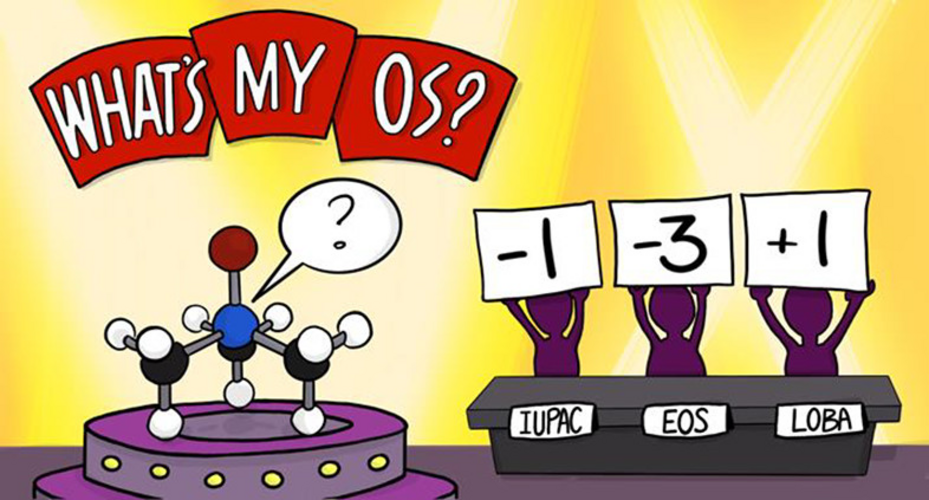
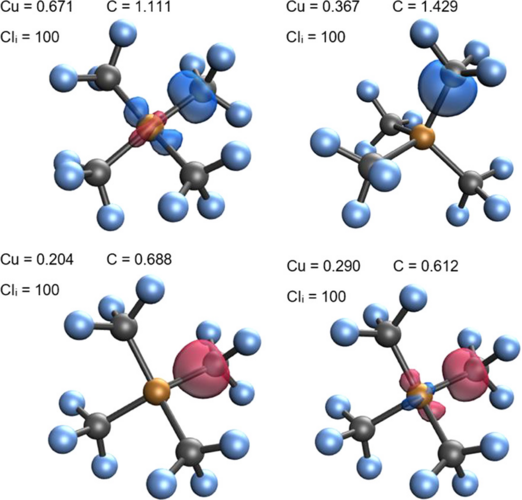
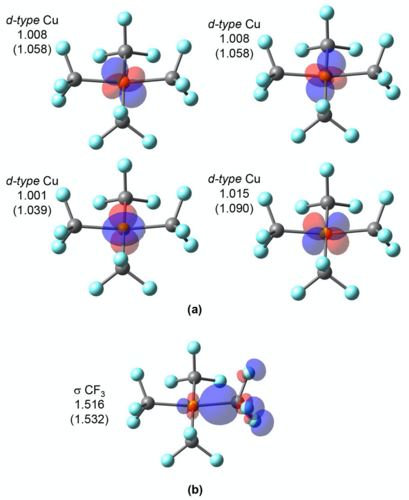
Oxidation state (OS) is one of the most fundamental chemical concepts in molecular reactivity studies. IUPAC defines OS as the atom’s charge after ionic approximation of its heteronuclear bonds. However, this winner-takes-all charge assignment scheme shows its limit in transition metal complexes (TMC) in non-ionic chemical environments.
-
Q-Chem offers two localized-orbital-based tools for assigning oxidation states: localized orbital bonding analysis (LOBA) and oxidation states from localized orbitals (OSLO).
-
In LOBA, a electron pair population analysis is done on localized orbitals. A clarity index (CI) is introduced to estimate how conclusive the OS assignment for nontrivial bonds would be.
-
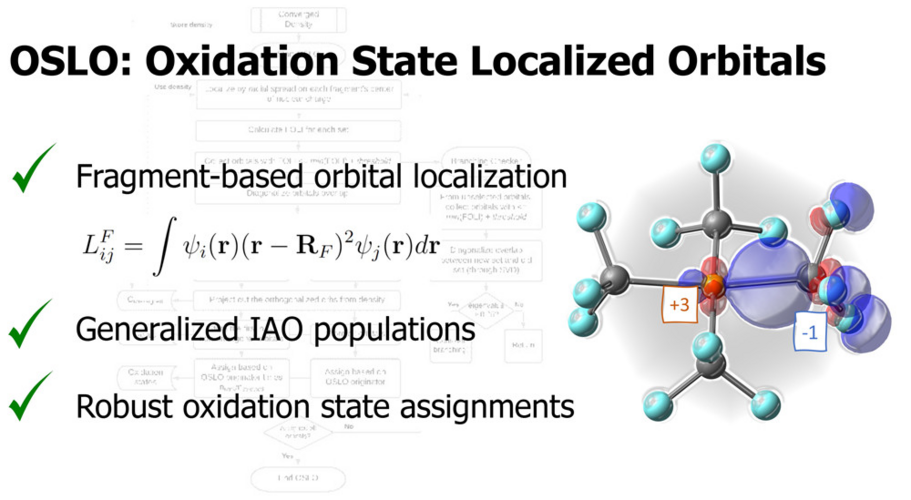
In OSLO, the orbitals localized to user-defined molecular fragments are used for population analysis. A fragment orbital localization index (FOLI) is introduced to tell how localized the orbital is on the fragment. Q-Chem also offers options to the user to choose population analysis approaches and branching schemes during the iterative OSLO calculation.
-
LOBA and OSLO can help chemists clarify borderline OS assignments in transition metal complexes.
Want to try Q-Chem?